Strengthening Aotearoa New Zealand’s shellfish industries through selective breeding
From wild spat to targeted genetic gain: Building the foundation for long-term success
November 2025
Our Expertise
At Cawthron, our aquaculture breeding team includes more than 20 researchers, geneticists, and technical specialists with deep expertise in hatchery systems, selective breeding, and aquatic species biology and husbandry. We work closely with industry partners to co-design and implement practical, scalable breeding solutions tailored to the needs of aquaculture industries in New Zealand and around the world. By combining science with real-world application, we’ve helped deliver measurable genetic gains, improved production performance, and strengthened the long-term sustainability and capability of Aotearoa New Zealand’s shellfish industry.
Background
Aotearoa New Zealand’s aquaculture industry relies on two iconic species: the Greenshell™ mussel (Perna canaliculus) and the Pacific oyster (Crassostrea gigas). Historically, both industries were 100 % dependent on wild-collected spat-subject to unpredictable supply and variable performance. With limited hatchery infrastructure, no structured breeding systems, and mounting production pressures, there was a clear need for a more controlled, reliable, consistent, and performance-focused approach.
Recognising this, Cawthron partnered with government and industry to initiate the co-development of novel hatchery techniques and breeding programmes for both species. Working closely with industry partners and supported by strategic government investment, these efforts laid the foundation for two very successful selective breeding pipelines in Aotearoa New Zealand aquaculture. Today, the impact is clear – selectively bred shellfish now represent a significant and growing proportion of the national harvest, delivering measurable improvements in growth, survival, and product quality.
Pacific Oyster: From survival crisis to commercial stability
Aotearoa New Zealand’s Pacific oyster industry initially relied on wild-collected spat, offering little consistency in performance and leaving the sector vulnerable to disease. The emergence of OsHV‑1 (Ostreid herpes virus) in the North of the country in 2010 triggered widespread mortality and financial losses. Breeding for virus resistance quickly became a priority.
Building the breeding programme
In 1999, Cawthron Institute in partnership with industry, launched the first structured oyster family breeding programme. Early efforts centred on developing family-based trials using CUDLS (Cawthron Ultra Density Larval System) tanks designed by Cawthron, breeding for faster growth and aesthetic qualities, and assessing growth performance under commercial conditions. As these trials generated robust trait data, the programme adopted genetic evaluation tools in 2010, including BLUP (Best Linear Unbiased Prediction) and ASReml software. This marked a turning point where families could now be selected based on genetic merit for OsHV‑1 survival, growth, and shell quality.
Evidence of genetic gain
Challenge trials under OsHV-1 exposure showed that survival rates in selected families were up to 50% higher than in unselected controls – strong proof that resistance is both heritable and selectable (Camara et al., 2017). Following on from this success, the breeding program then started to incorporate multiple traits beyond survival, including shell shape and growth (Camara and Symonds 2014). Growth rate and uniformity further improved, resulting in more marketable spat and oysters.
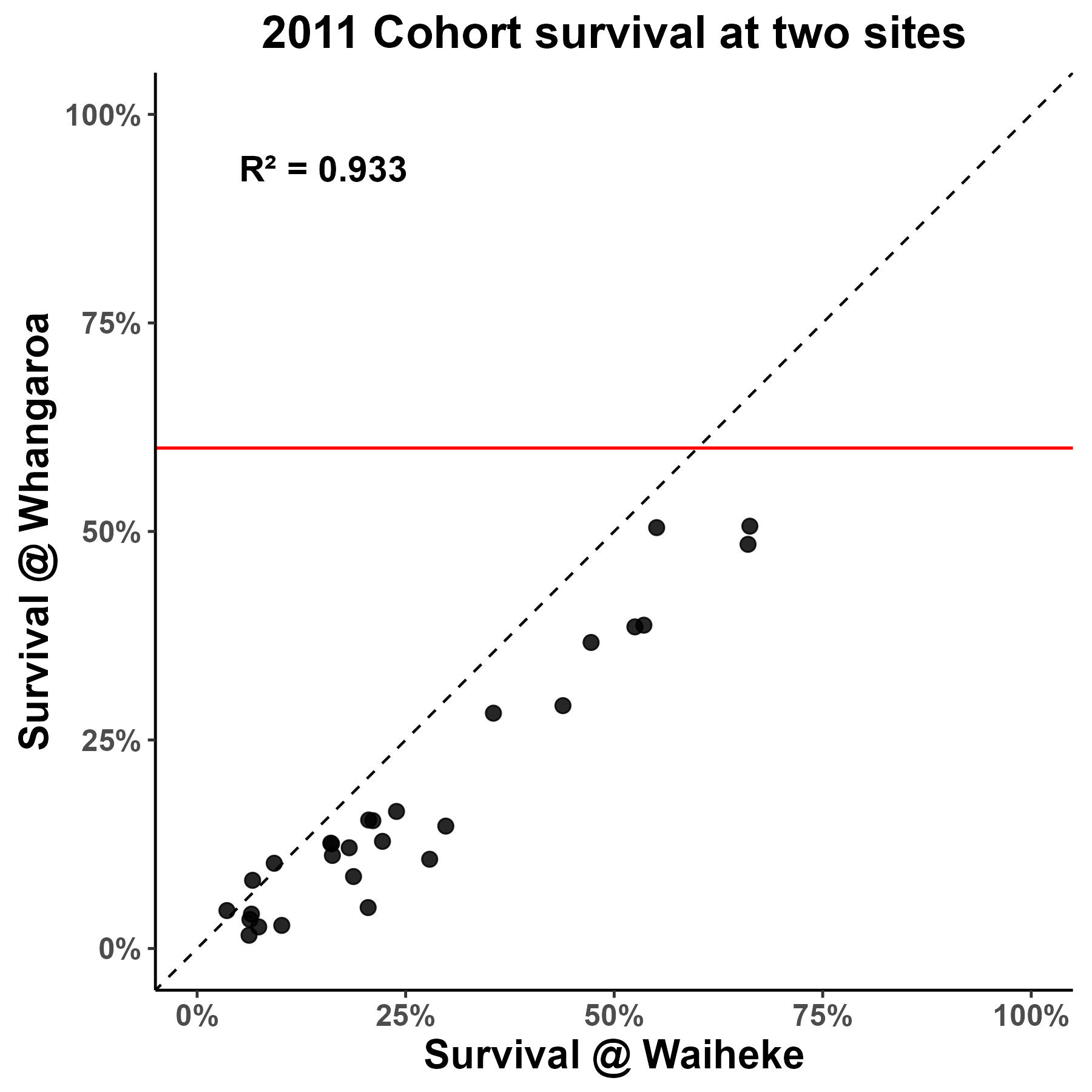
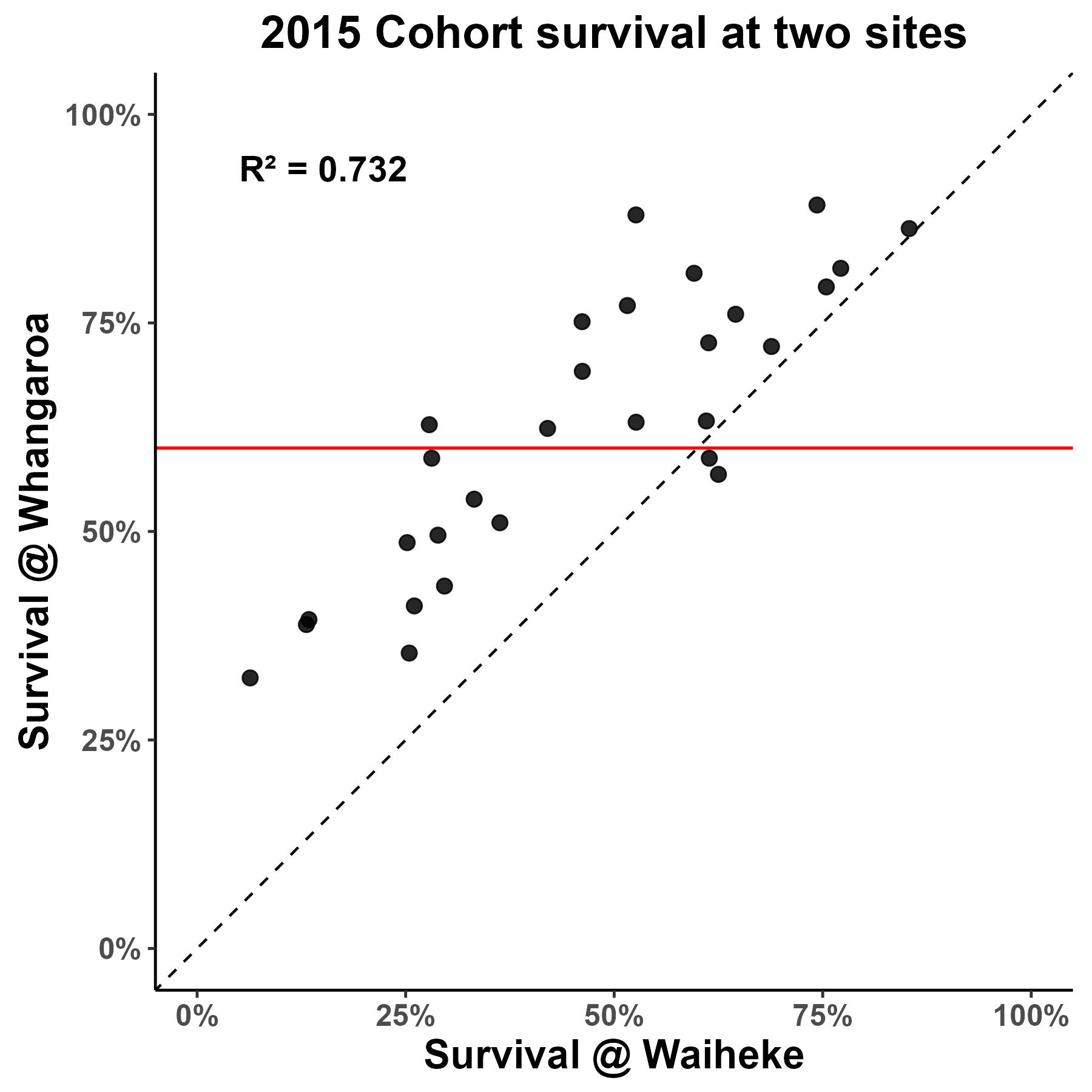
Figure 2. Family field survival between farm sites for the 2011 cohort and the 2015 cohort which was selectively bred for virus resilience. You can clearly see the improvement on field survival of families selectively bred for virus resilience/ genetic gain between the two cohorts. For example, at Whangaroa no families from the 2011 cohort showed field survival above 60%, whilst more than half of the families from the 2015 cohort showed field survival above 60%.
Enhancing Oyster Production with Triploidy
Cawthron has been at the forefront of triploid oyster production, offering clients year-round spat supply with improved meat quality, and predictable harvest cycles since 2010. Triploid Pacific oysters produced via chemical induction or tetraploid mating, outperformed diploids in growth and flesh yield while remaining functionally sterile (Vignier et al., 2025).
That study reported >95% triploid status, superior meat yield, and extended harvest potential with no trade-offs in OsHV-1 susceptibility or high temperature tolerance.
Triploidy complements selective breeding by enabling continuous harvest windows and aligning product with premium market schedules, enhancing commercial flexibility and profitability.

Commercial uptake & broader research context
Today, almost 60% of New Zealand’s Pacific oyster production relies on selectively bred hatchery triploid spat using Cawthron Aquaculture and Breeding Services (CABS). This is a dramatic shift from the wild-only supply of two decades ago. Moana New Zealand’s hatchery and the Cawthron Aquaculture Park hatchery and nursery facilities now support year-round production, ensuring reliable delivery of high-performance spat to growers.
Parallel studies, including laboratory and field challenges, have reinforced the value of selective breeding (Camara et al., 2017). Delisle et al. (2022) demonstrated that OsHV‑1 resistant breeding lines showed distinct microbiome profiles-suggesting that disease resistance may be influenced by both host genetics and microbial interactions. Camara and Symonds (2014) noted early-stage success in multi-trait selection and more recent studies show the promise of genomic selection to accelerate gains (Gutierrez et al., 2020).
Future directions
Looking forward, Cawthron is exploring genomic selection to enhance accuracy and speed up deployment of improved lines. Triploid technology is under evaluation to further determine the interactions of environmental conditions and genetics (GxE) to deliver consistent meat yield and harvest scheduling advantages. Other ongoing research aims to unravel the mechanisms of host–pathogen-environment interactions that underpin disease resistance and climate resilience to better develop complementary strategies to mitigate these risks.
Greenshell™ Mussel: Building genetic infrastructure from the ground up
Prior to the early 2000s, the Greenshell™ mussel (Perna canaliculus) industry in Aotearoa New Zealand relied entirely on wild-collected spat, offering little control over growth, survival, or seasonality. The variability of wild spat supply, often dependent on unpredictable beach cast events, led to inconsistent farm performance and vulnerability to climatic stressors. Recognising these issues, Cawthron Institute partnered with industry and government to form Aotearoa New Zealand’s first structured mussel breeding programme in 2002. Working alongside SPATnz, BreedCo, and other key partners, this initiative laid the scientific and operational groundwork for long-term genetic improvement.
Building the Breeding Programme
Initial programme steps included establishing family lines using CUDLS and running replicated grow-out trials across multiple sites to evaluate growth and condition metrics.

This confirmed heritable variation in key traits and informed selection goals. A pivotal moment occurred in 2015 with the development of the SPATnz pilot-scale hatchery, funded by the Ministry for Primary Industries through the Primary Growth Partnership, which allowed for large-scale family rearing, individual tagging, and performance tracking (MPI 2019, Sanford 2019). Since then, infrastructure has been scaled to support cohorts typically exceeding one million juveniles, and selection strategies are repeated across generations using phenotype-based and pedigree-informed data.
Evidence of Genetic Gain
Performance improvements in selectively bred mussels emerged rapidly. Hatchery-reared lines achieved up to twice the growth rate of wild-caught spat under comparable commercial conditions, reaching harvest size approximately 12 months sooner (16.7 vs. 28.3 months), which drastically improves production efficiency (MPI 2019, Sanford 2019). These gains align with global expectations for selective breeding; well-managed programmes typically achieve 12–15% improvement per generation in growth traits (Camara and Symonds 2014). Family-level analyses confirmed significant variation in condition index and summer survival – key traits targeted through selection.
Commercial Uptake & Scaling
Today, selectively bred Greenshell™ mussels are on track to supply around 40% of Aotearoa’s New Zealand’s national production, with hatchery capacity being scaled up to meet growing demand. These mussels deliver faster growth, improved condition, and greater production predictability, benefits that cascade through the supply chain, from seed growers to export-ready product.
The commercial and economic impact is substantial: by providing a scalable seed supply to underpin expansion and increasing the productivity of existing farms, hatchery mussels in Aotearoa New Zealand could deliver NZD $200 million in annual value gains (MPI 2019, Sanford 2019).
Future Directions
With a solid breeding platform in place, Greenshell™ mussel breeding is now embracing advanced technologies.
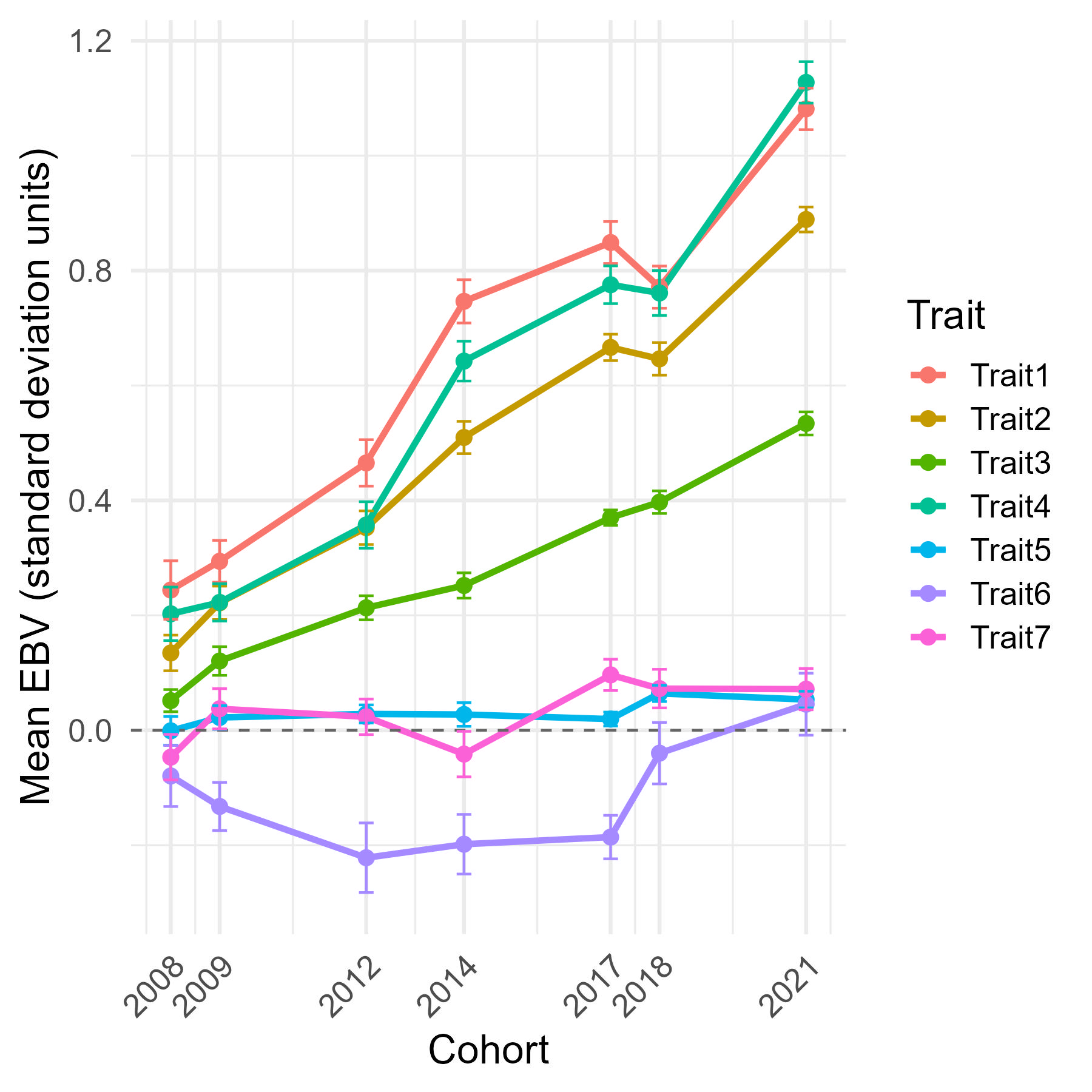
Figure 4. Genetic trends of key Greenshell mussel traits, calculated as the mean EBV of the top 50 families per cohort. Trait gains vary with annual selection emphasis: traits 1-4 show consistent improvement (~ +1 SD from the 2008 base), whereas traits 5 & 7 show little change, consistent with the aim of maintaining, rather than enhancing, these traits.
There is ongoing research on thermotolerance and physiological biomarkers to support breeding for climate resilience and ensuring future-tested broodstock (Ericson et al., 2024; Scholtens et al., 2025; Symonds et al., 2025).
This journey from reliance on wild spat to genomic-informed breeding, is a testament to the power of combined science, infrastructure, and industry collaboration. As selective breeding becomes the norm, Aotearoa New Zealand’s Greenshell™ mussel industry is positioned for sustainable, efficient, and profitable growth.
Summary
What Made These Programmes Successful
The success of these programmes was not driven by genetics alone. From the outset, the approach was practical, industry-led, and focused on outcomes. Breeding goals were co-designed with farmers and processors to ensure relevance. Trial designs and data systems were built to support decision-making at scale, and early gains were clearly demonstrated in on-farm conditions. Government support during the capability-building phase was critical, as was the willingness of pioneer industry partners to invest in infrastructure and participation in trials.
Perhaps most importantly, the programmes were built to deliver. Rather than focusing solely on research outcomes, the breeding pipelines were structured around commercial deployment, ensuring that gains could be realised on the water and in the marketplace.
A Model for the Future
The Greenshell™ mussel and Pacific oyster breeding programmes are now considered essential components of Aotearoa New Zealand’s aquaculture sector. Their success demonstrates the power of partnership, strategic investment, and science-led delivery to drive real-world impact. With genomic tools being introduced and hatchery capacity continuing to expand, the industry is well-positioned to accelerate further gains in resilience, quality, and productivity.
Bespoke Solutions to Meet Individual Needs
At Cawthron, we specialise in providing customised breeding and hatchery solutions designed to meet the specific needs of individual production systems. “We work closely with clients to provide the best solutions for their needs, including several partnerships to co-develop tailored scale-up hatchery and breeding designs,” explains Dr Seumas Walker, Manager of Cawthron’s Aquaculture Group. This client-focused approach is coupled with world-leading technical expertise, which has already designed some of the industry’s most widely used larval rearing systems (CUDLS).

Figure: Cawthron Ultra Density Larval System (CUDLS) for creating breeding families.
References:
Delisle, L., Laroche, O., Hilton, Z., Burguin, J. F., Rolton, A., Berry, J., … & Vignier, J. (2022). Understanding the dynamic of POMS infection and the role of microbiota composition in the survival of Pacific oysters, Crassostrea gigas. Microbiology Spectrum, 10(6), e01959-22.
Dunphy, B. J., Ragg, N. L., & Collings, M. G. (2013). Latitudinal comparison of thermotolerance and HSP70 production in F2 larvae of the greenshell mussel (Perna canaliculus). Journal of Experimental Biology, 216(7), 1202-1209.
Ericson, J. A., Laroche, O., Biessy, L., Delorme, N. J., Pochon, X., Thomson-Laing, J., … & Smith, K. F. (2024). Differential responses of selectively bred mussels (Perna canaliculus) to heat stress—survival, immunology, gene expression and microbiome diversity. Frontiers in Physiology, 14, 1265879.
Gutierrez, A. P., Symonds, J., King, N., Steiner, K., Bean, T. P., & Houston, R. D. (2020). Potential of genomic selection for improvement of resistance to ostreid herpesvirus in Pacific oyster (Crassostrea gigas). Animal genetics, 51(2), 249-257.
Hess, A. S., Dodds, K. G., Roberts, R. D., King, N., Symonds, J., & Clarke, S. M. (2018). Genetic parameters of GreenshellTM mussel traits under two harvest seasons.
Camara, M. D., & Symonds, J. E. (2014). Genetic improvement of New Zealand aquaculture species: programmes, progress and prospects. New Zealand Journal of Marine and Freshwater Research, 48(3), 466-491.
Camara, M.D., S. Yen, H.F. Kaspar, A. Kesarcodi-Watson, N. King et al., 2017 Assessment of heat shock and laboratory virus challenges to selectively breed for ostreid herpesvirus 1 (OsHV-1) resistance in the Pacific oyster, Crassostrea gigas. Aquaculture 469:50-58.
MPI 2019. Startling trial results for Greenshell mussel breeding | NZ Government
Sanford 2019. SPATnz-RESULTS-REVEAL-18-OCT-2019-PRESS-RELEASE.pdf
Scholtens, M. R., Roberts, R., Delorme, N. J., King, N., Ragg, N. L. C., Costilla, R., & Ericson, J. A. Estimating thermal tolerance in selectively-bred New Zealand Greenshell mussels (Perna Canaliculus). (2025). In Proc. Assoc. Advmt. Anim. Breed. Genet (Vol. 26, pp. 114-117).
Symonds, J., Scholtens, M., Dodds, K., Clarke, S., Costilla, R., Walker, S., … & Slattery, T. (2025). Growing Aotearoa New Zealand’s aquaculture industry through breeding and genomics: progress, challenges and future opportunities. In Proc. Assoc. Advmt. Anim. Breed. Genet (Vol. 26, pp. 6-12).
Symonds, J. E., Clarke, S. M., King, N., Walker, S. P., Blanchard, B., Sutherland, D., … & Dodds, K. G. (2019). Developing successful breeding programs for New Zealand aquaculture: a perspective on progress and future genomic opportunities. Frontiers in Genetics, 10, 27.
Vignier, J., Reardon, M., Exton, M., Delisle, L., Rolton, A., Malpot, E., … & Adams, S. (2025). Comparative performance of selected triploid oysters Crassostrea (Magallana) gigas, produced by chemical induction and mated triploid techniques, to their diploid counterparts. Aquaculture, 596, 741894.
Contact us

Jane Symonds
Team Leader - Finfish Aquaculture

Megan Scholtens
Aquaculture Scientist

Seumas Walker
Aquaculture Group Manager
